Utilisateur:Matrok2/Brouillon
- → N'hésitez pas à publier sur le brouillon un texte inachevé et à le modifier autant que vous le souhaitez.
- → Pour enregistrer vos modifications au brouillon, il est nécessaire de cliquer sur le bouton bleu : « Publier les modifications ». Il n'y a pas d'enregistrement automatique.
Si votre but est de publier un nouvel article, votre brouillon doit respecter les points suivants :
- Respectez le droit d'auteur en créant un texte spécialement pour Wikipédia en français (pas de copier-coller venu d'ailleurs).
- Indiquez les éléments démontrant la notoriété du sujet (aide).
- Liez chaque fait présenté à une source de qualité (quelles sources – comment les insérer).
- Utilisez un ton neutre, qui ne soit ni orienté ni publicitaire (aide).
- Veillez également à structurer votre article, de manière à ce qu'il soit conforme aux autres pages de l'encyclopédie (structurer – mettre en page).
- → Si ces points sont respectés, pour transformer votre brouillon en article, utilisez le bouton « publier le brouillon » en haut à droite. Votre brouillon sera alors transféré dans l'espace encyclopédique.
Les diagrames de Tanabe–Sugano sont utilisés en chimie de coordination pour prédire l'absorption dans le spectre électromagnetique UV, visible et IR d'un complexe de coordination. Le résultat de l'analyse d'un diagramme de Tanabe–Sugano d'un complexe métallique peut aussi être comparé aux données spectroscopiques experimentales. Ces diagrammes sont utiles d'un point de vue qualitatif et peuvent être utilisés pour approximer la valeur de 10Dq, l'énergie d'éclatement du champ des ligands. Ils peuvent être utilisés aussi ien pour les complexes à bas spin qu'à haut spin, contrairement aux diagrammes d'Orgel qui ne s'appliquent qu'aux complexes à haut spin. Les diagrammes de Tanabe–Sugano peuvent aussi être utilisés pour prédire la taille du champ de ligand nécessaire pour causer une transition de haut spin à bas spin.
Dans un diagramme de Tanabe–Sugano, l'état fondamental est utilisé comme une référence constante, contrairement aux diagrammes d'Orgel. L'énergie de l'état fondamental est prise égale à zéro pour toutes les forces de champ, et l'énergie des autres termes et de leurs composants sont tracées par rapport à l'état fondamental.
Historique[modifier | modifier le code]
Avant que Yukito Tanabe et Satoru Sugano publient leur article intitulé On the absorption spectra of complex ions, les états électroniques excités des complexes des ions métalliques étaient mal connus. Ils utilisèrent la théorie du champ cristallin de Hans Bethe et les combinaisons d'intégrales de Slater de Giulio Racah[1] qu'on appelle aujourd'hui paramètre de Racah, pour expliquer les spectres d'absorption des complexes octahédriques d'une façon plus quantitative que ce qui avait été fait jusque là.[2] Après de nombreuses expériences de spectroscopie, ils estimèrent les valeurs de deux des paramètres de Racah, B and C, pour chacune des configurations électroniques des électrons d à partir des tendances dans les spectres d'absorption des complexes isoélectroniques des métaux de transition de la première période. C'est le tracé des énergies calculées pour les états électroniques de chaque configuration électronique qu'on appelle maintenant diagrammes de Tanabe-Sugano[3],[4].
Paramètres[modifier | modifier le code]
L'axe des abscisses d'un diagramme de Tanabe–Sugano représente le paramètre d'éclatement du champ des ligands, Dq, or Δ, divisé par le paramètre de Racah B. L'axe des ordonnées représente l'énergie E, là encore divisée par B. Il existe trois paramètres de Racah, A, B, et C, qui décrivent différents aspects de la répulsion interélectronique. A est une moyenne de la répulsion interélectronique totale. B et C correspondent aux répulsions individuelles entre électrons d. A est constante pour une configuration des électrons d, et n'est pas nécessaire pour calculer les énergies relatives, d'où son absence de l'étude des ions complexes par Tanabe et Sugano. C ne sert que dans certains cas. B est le plus important des paramètres de Racah dans ce cas.[5] One line corresponds to each electronic state. The bending of certain lines is due to the mixing of terms with the same symmetry. Although electronic transitions are only "allowed" if the spin multiplicity remains the same (i.e. electrons do not change from spin up to spin down or vice versa when moving from one energy level to another), energy levels for "spin-forbidden" electronic states are included in the diagrams, which are also not included in Orgel diagrams.[6] Each state is given its symmetry label (e.g. A1g, T2g, etc.), but "g" and "u" subscripts are usually left off because it is understood that all the states are gerade. Labels for each state are usually written on the right side of the table, though for more complicated diagrams (e.g. d6) labels may be written in other locations for clarity. Term symbols (e.g. 3P, 1S, etc.) for a specific dn free ion are listed, in order of increasing energy, on the y-axis of the diagram. The relative order of energies is determined using Hund's rules. For an octahedral complex, the spherical, free ion term symbols split accordingly:[7]
| Term | Degeneracy | States in an octahedral field |
|---|---|---|
| S | 1 | A1g |
| P | 3 | T1g |
| D | 5 | Eg + T2g |
| F | 7 | A2g + T1g + T2g |
| G | 9 | A1g + Eg + T1g + T2g |
| H | 11 | Eg + T1g + T1g + T2g |
| I | 13 | A1g + A2g + Eg + T1g + T2g + T2g |
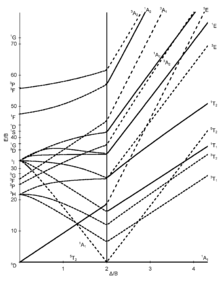
Certain Tanabe–Sugano diagrams (d4, d5, d6, and d7) also have a vertical line drawn at a specific Dq/B value, which corresponds with a discontinuity in the slopes of the excited states' energy levels. This pucker in the lines occurs when the spin pairing energy, P, is equal to the ligand field splitting energy, Dq. Complexes to the left of this line (lower Dq/B values) are high-spin, while complexes to the right (higher Dq/B values) are low-spin. There is no low-spin or high-spin designation for d2, d3, or d8[8].
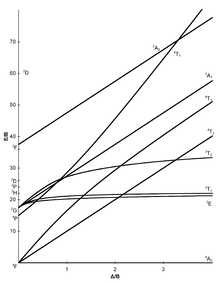
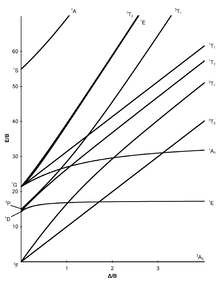
Tanabe-Sugano diagrams[modifier | modifier le code]
The seven Tanabe–Sugano diagrams for octahedral complexes are shown below.[5][9][10]
 |
 |
 |
 |
 |
 |
 |
Unnecessary diagrams: d1, d9 and d10[modifier | modifier le code]
d1[modifier | modifier le code]
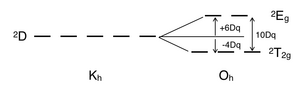
There is no electron repulsion in a d1 complex, and the single electron resides in the t2g orbital ground state. A d1 octahedral metal complex, such as [Ti(H2O)6]3+, shows a single absorption band in a UV-vis experiment.[5] The term symbol for d1 is 2D, which splits into the 2T2g and 2Eg states. The t2g orbital set holds the single electron and has a 2T2g state energy of -4Dq. When that electron is promoted to an eg orbital, it is excited to the 2Eg state energy, +6Dq. This is in accordance with the single absorption band in a UV-vis experiment. The prominent shoulder in this absorption band is due to a Jahn-Teller distortion which removes the degeneracy of the two 2Eg states. However, since these two transitions overlap in a UV-vis spectrum, this transition from 2T2g to 2Eg does not require a Tanabe–Sugano diagram.
d9[modifier | modifier le code]
Similar to d1 metal complexes, d9 octahedral metal complexes have 2D spectral term. The transition is from the (t2g)6(eg)3 configuration (2Eg state) to the (t2g)5(eg)4 configuration (2T2g state). This could also be described as a positive "hole" that moves from the eg to the t2g orbital set. The sign of Dq is opposite that for d1, with a 2Eg ground state and a 2T2g excited state. Like the d1 case, d9 octahedral complexes do not require the Tanabe–Sugano diagram to predict their absorption spectra.
d10[modifier | modifier le code]
There are no d-d electron transitions in d10 metal complexes because the d orbitals are completely filled. Thus, UV-vis absorption bands are not observed and a Tanabe–Sugano diagram does not exist.
Diagrams for tetrahedral symmetry[modifier | modifier le code]
Tetrahedral Tanabe–Sugano diagrams are generally not found in textbooks because the diagram for a dn tetrahedral will be similar to that for d(10-n) octahedral, remembering that ΔT for tetrahedral complexes is approximately 4/9 of ΔO for an octahedral complex. A consequence of the much smaller size of ΔT results in (almost) all tetrahedral complexes being high spin and therefore the change in the ground state term seen on the X-axis for octahedral d4-d7 diagrams is not required for interpreting spectra of tetrahedral complexes.
Advantages over Orgel diagrams[modifier | modifier le code]
In Orgel diagrams, the magnitude of the splitting energy exerted by the ligands on d orbitals, as a free ion approach a ligand field, is compared to the electron-repulsion energy, which are both sufficient at providing the placement of electrons. However, if the ligand field splitting energy, 10Dq, is greater than the electron-repulsion energy, then Orgel diagrams fail in determining electron placement. In this case, Orgel diagrams are restricted to only high spin complexes[6].
Tanabe–Sugano diagrams do not have this restriction, and can be applied to situations when 10Dq is significantly greater than electron repulsion. Thus, Tanabe–Sugano diagrams are utilized in determining electron placements for high spin and low spin metal complexes. However, they are limited in that they have only qualitative significance. Even so, Tanabe–Sugano diagrams are useful in interpreting UV-vis spectra and determining the value of 10Dq[6].
Applications as a qualitative tool[modifier | modifier le code]
In a centrosymmetric ligand field, such as in octahedral complexes of transition metals, the arrangement of electrons in the d-orbital is not only limited by electron repulsion energy, but it is also related to the splitting of the orbitals due to the ligand field. This leads to many more electron configuration states than is the case for the free ion. The relative energy of the repulsion energy and splitting energy defines the high-spin and low-spin states.
Considering both weak and strong ligand fields, a Tanabe–Sugano diagram shows the energy splitting of the spectral terms with the increase of the ligand field strength. It is possible for us to understand how the energy of the different configuration states is distributed at certain ligand strengths. The restriction of the spin selection rule makes it is even easier to predict the possible transitions and their relative intensity. Although they are qualitative, Tanabe–Sugano diagrams are very useful tools for analyzing UV-vis spectra: they are used to assign bands and calculate Dq values for ligand field splitting[11],[12].
Examples[modifier | modifier le code]

Manganese(II) hexahydrate[modifier | modifier le code]
In the [Mn(H2O)6]2+ metal complex, manganese has an oxidation state of +2, thus it is a d5 ion. H2O is a weak field ligand (spectrum shown below), and according to the Tanabe–Sugano diagram for d5 ions, the ground state is 6A1. Note that there is no sextet spin multiplicity in any excited state, hence the transitions from this ground state are expected to be spin-forbidden and the band intensities should be low. From the spectra, only very low intensity bands are observed (low Molar absorptivity (ε) values on y-axis)[11].
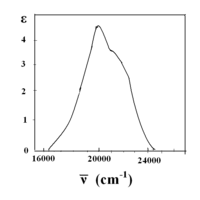
Cobalt(II) hexahydrate[modifier | modifier le code]
Another example is [Co(H2O)6]2+.[12] Note that the ligand is the same as the last example. Here the cobalt ion has the oxidation state of +2, and it is a d7 ion. From the high-spin (left) side of the d7 Tanabe–Sugano diagram, the ground state is 4T1(F), and the spin multiplicity is a quartet. The diagram shows that there are three quartet excited states: 4T2, 4A2, and 4T1(P). From the diagram one can predict that there are three spin-allowed transitions. However, the spectra of [Co(H2O)6]2+ does not show three distinct peaks that correspond to the three predicted excited states. Instead, the spectrum has a broad peak (spectrum shown below). Based on the T-S diagram, the lowest energy transition is 4T1 to 4T2, which is seen in the near IR and is not observed in the visible spectrum. The main peak is the energy transition 4T1(F) to 4T1(P), and the slightly higher energy transition (the shoulder) is predicted to be 4T1 to 4A2. The small energy difference leads to the overlap of the two peaks, which explains the broad peak observed in the visible spectrum.
Solving for B and ΔO[modifier | modifier le code]

For the d2 complex [V(H2O)6]3+, two bands are observed with maxima at around 17,500 and 26,000 cm−1.[réf. nécessaire] The ratio of experimental band energies is E(ν2)/E(ν1) is 1.49. There are three possible transitions expected, which include: ν1: 3T1g→3T2g, ν2:3T1g→3T1g(P), and ν3: 3T1g→3A2g. There are three possible transitions, but only two are observed, so the unobserved transition must be determined.
| ΔO / B = | 10 | 20 | 30 | 40 |
|---|---|---|---|---|
| Height E(ν1)/B | 10 | 19 | 28 | 37 |
| Height E(ν2)/B | 23 | 33 | 42 | 52 |
| Height E(ν3)/B | 19 | 38 | 56 | 75 |
| Ratio E(ν3)/E(ν1) | 1.9 | 2.0 | 2.0 | 2.0 |
| Ratio E(ν2)/E(ν1) | 2.3 | 1.73 | 1.5 | 1.4 |
Fill in a chart like the one to the right by finding corresponding heights (E/B) of the symmetry states at certain values of ΔO / B. Then find the ratio of these values (E(ν2)/E(ν1) and E(ν3)/E(ν1)). Note that the ratio of E(ν3)/E(ν1) does not contain the calculated ratio for the experimental band energy, so we can determine that the 3T1g→3A2g band is unobserved. Use ratios for E(ν2)/E(ν1) and the values of ΔO / B to plot a line with E(ν2)/E(ν1) being the y-values and ΔO/B being the x-values. Using this line, it is possible to determine the value of ΔO / B for the experimental ratio. (ΔO / B = 31 for a chart ratio of 1.49 in this example).
Find on the T-S diagram where ΔO / B = 31 for 3T1g→3T2g and 3T1g→3T1g(P). For 3T2g, E(ν1) / B = 27 and for 3T1g(P), E(ν2) / B = 43.
The Racah parameter can be found by calculating B from both E(ν2) and E(ν1). For 3T1g(P), B = 26,000 cm−1/43 = 604 cm−1. For 3T2g, B = 17,500 cm−1/ 27 = 648 cm−1. From the average value of the Racah parameter, the ligand field splitting parameter can be found (ΔO). If ΔO / B = 31 and B = 625 cm−1, then ΔO = 19,375 cm−1.
See also[modifier | modifier le code]
- Character tables
- Crystal field theory
- d electron count
- Hans Bethe
- Laporte rule
- Ligand field theory
- Molecular symmetry
- Orgel diagram
- Racah parameter
- Spin states (d electrons)
- Term symbol
References[modifier | modifier le code]
- Giulio Racah, « Theory of complex spectra II », Physical Review, vol. 62, nos 9–10, , p. 438–462 (DOI 10.1103/PhysRev.62.438, Bibcode 1942PhRv...62..438R)
- Yukito Tanabe et Satoru Sugano, « On the absorption spectra of complex ions I », Journal of the Physical Society of Japan, vol. 9, no 5, , p. 753–766 (DOI 10.1143/JPSJ.9.753)
- Yukito Tanabe et Satoru Sugano, « On the absorption spectra of complex ions II », Journal of the Physical Society of Japan, vol. 9, no 5, , p. 766–779 (DOI 10.1143/JPSJ.9.766)
- Yukito Tanabe et Satoru Sugano, « On the absorption spectra of complex ions III », Journal of the Physical Society of Japan, vol. 11, no 8, , p. 864–877 (DOI 10.1143/JPSJ.11.864)
- (en) Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, Fraser Armstrong, Paul Salvador, Michael Hagerman, Thomas Spiro et Edward Stiefel, Shriver & Atkins Inorganic Chemistry, New York, 4th, , 478–483 p. (ISBN 0-7167-4878-9)
- (en) Bodie Douglas, Darl McDaniel et John Alexander, Concepts and Models of Inorganic Chemistry, New York, 3rd, , 442–458 p. (ISBN 0-471-62978-2)
- (en) F. Albert Cotton, Geoffrey Wilkinson et Paul L. Gaus, Basic Inorganic Chemistry, New York, 3rd, , 530–537 p. (ISBN 0-471-50532-3)
- (en) Daniel C. Harris et Michael D. Bertolucci, Symmetry and Spectroscopy: An Introduction to Vibrational and Electronic Spectroscopy, New York, Dover Publications, Inc., , 403–409, 539 (ISBN 978-0-486-66144-5)
- « {{{1}}} »
- Robert John Lancashire, « Tanabe-Sugano diagrams via spreadsheets », (consulté le )
- Chr Klixbüll Jørgensen, Carl-Henric De Verdier, John Glomset et Nils Andreas Sörensen, « Studies of absorption spectra IV: Some new transition group bands of low intensity », Acta Chem. Scand., vol. 8, no 9, , p. 1502–1512 (DOI 10.3891/acta.chem.scand.08-1502)
- Chr Klixbüll Jørgensen, Carl-Henric De Verdier, John Glomset et Nils Andreas Sörensen, « Studies of absorption spectra III: Absoprtion Bands as Gaussian Error Curves », Acta Chem. Scand., vol. 8, no 9, , p. 1495–1501 (DOI 10.3891/acta.chem.scand.08-1495)
{{DEFAULTSORT:Tanabe-Sugano diagram}} [[Category:Coordination chemistry]] [[Category:Spectroscopy]] [[Category:Inorganic chemistry]] [[Category:Transition metals]]